Reinstating Trust in the COVID-19 Testing Process
(BPT) - Since the World Health Organization (WHO) declared the rapidly spreading coronavirus outbreak a pandemic on March 11, 2020, the Centers for Disease Control and Prevention (CDC) and U.S. Food and Drug Administration (FDA) needed to act at an incredible speed to determine how much of the U.S. population was infected and measure how fast the virus was spreading. To rapidly make widespread testing available, the FDA put into place the emergency use authorization (EUA) process that allowed laboratories and commercial manufacturers to accelerate the use of tests they developed, and provide confidence that the tests meet the FDA’s performance and data examinations. To date, the FDA has issued more than 100 EUAs for all types of coronavirus test kits.
“Our familiarity with the FDA’s regulation and validation processes prompted us to swiftly produce antibody tests that would meet its rigorous EUA criteria,” said Chockalingam Palaniappan, Ph.D., chief innovation officer at Ortho Clinical Diagnostics. “We wanted to act quickly, but we needed to do so with the utmost level of responsibility for patients, ensuring the tests met the highest accuracy standards. We applied all we learned over the past 35 years of providing critical clinical diagnostic information in response to infectious disease outbreaks, like HIV, to this test to demonstrate we were providing the most reliable results for physicians and patients. At Ortho, we believe every test is a life.”
What are the Two Types of Tests for SARS-CoV-2?
Viral RNA tests, also referred to as molecular or RT-PCR, were the first type developed for COVID-19, and were immediately used by the WHO and the CDC to detect the presence of viral genetic material. A positive result indicates an active COVID-19 infection. The tests do not rule out other bacterial infections or co-infections with other viruses like a cold or flu and can be inaccurate.
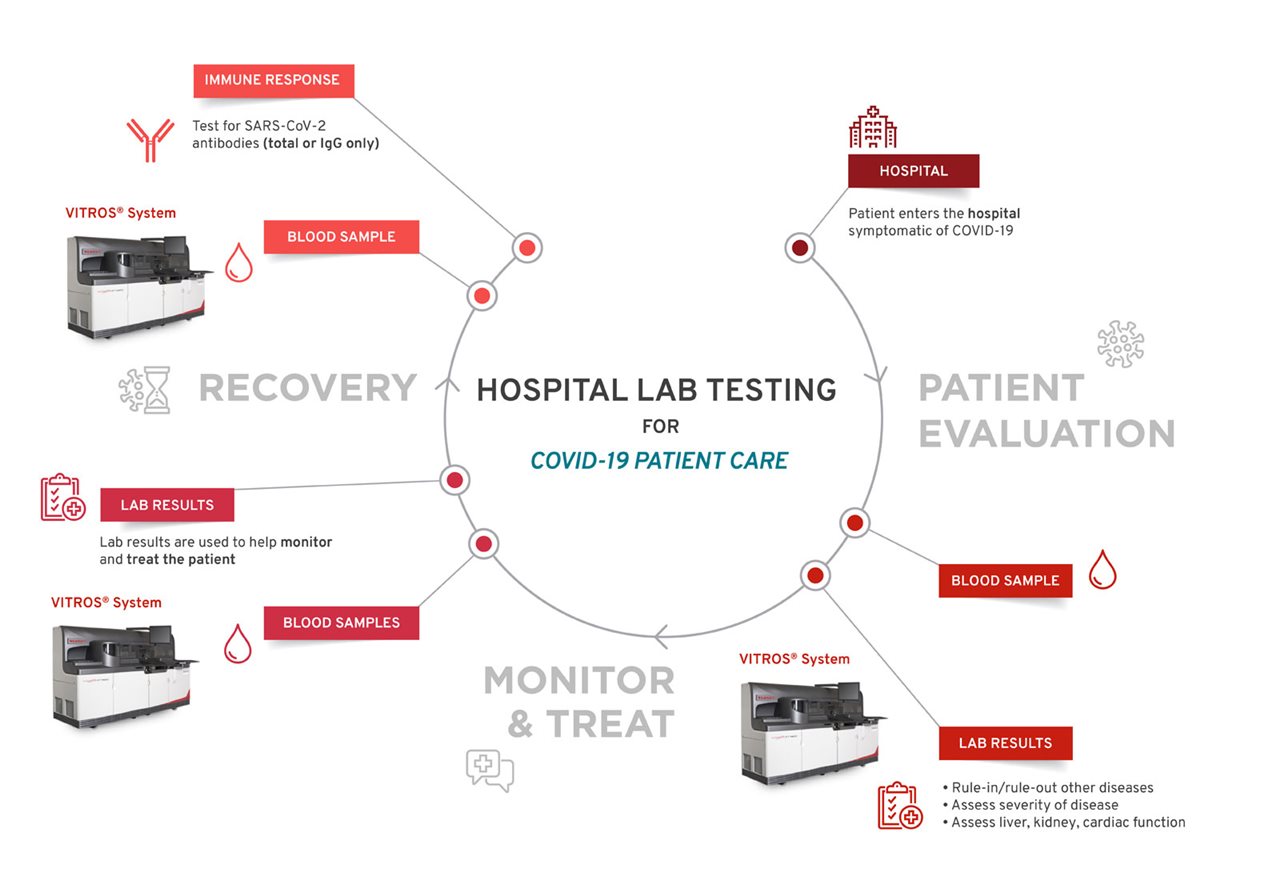
Antibody tests, also called serology tests, can identify individuals who have developed an immune response to COVID-19. These tests can aid in diagnosing symptomatic or asymptomatic individuals when combined with molecular tests and provide other clinical information. They can also identify individuals who have previously been infected with COVID-19 and recovered. High-throughput antibody testing, which are tests that can run on instruments that can handle many at once, are more accurate and better designed to handle high demand.
Once a patient is admitted into a hospital, their blood sample is collected and tested for antibodies, which can indicate an immune response, and may help clinicians to better manage the patient.
“It’s possible that people had COVID-19 without ever having symptoms, making it important to test for an immune response to the virus,” said Dr. Palaniappan. “People want to go back to work and restart the economy, and antibody testing is a tool that can help begin some of those discussions.”
Are there tests people can trust?
The FDA’s recent policy update on antibody tests for COVID-19 illustrated a crucial effort to reinstate the balance between supply, reassurance needs and quality, and corrected any misunderstanding about which tests are authorized for use. To date, less than 20 antibody tests have been granted FDA EUA. The FDA’s tightened restrictions on all antibody tests were a result of unreliable point-of-care tests flooding the market and “actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety.”[1] It’s important for all clinicians and public health officials to note the differences between rapid point-of-care, which may rely on a finger-prick of blood but have low accuracy, and high-throughput lab-based antibody tests.
As testing demand and priorities fluctuate, safety regulations must remain top of mind in order to best serve at-risk health care workers, hospitalized patients and, eventually, all citizens.
“Ortho has stepped up to offer reliable solutions at the global forefront of research and development of infectious disease diagnostics for over 35 years,” said Dr. Palaniappan. “We look forward to continuing to provide accurate and actionable data, especially in times of crisis.”
Real-world experiences, like data from states that are beginning to partially reopen, will continue to shape the COVID-19 testing landscape. Manufacturers, clinicians, lab professionals and local and state health departments should all take actions to collaborate as openly as possible to ensure a balanced approach in viral and antibody test development.
Patients should speak to their doctors to learn if antibody tests are right for them and visit the CDC website to learn how testing can affect them.